PhD
Thesis at PC2A depends on the doctoral (ED 104) SMRE Science de la Matière, du Rayonnement et de l'Environnement of the University of Lille.
PhD student : Debendra Behera
Thesis supervision : Florent Louis
Thesis co-supervisor : Pierre Herckes (Arizona State University)
Co-encadrement de thèse : Sonia Taamalli
Financial support :
Thesis start : February 2026
Context
The presence of emerging contaminants results either from direct emission or from diffuse emissions or re-emissions from contaminated soils or waterbodies. This phenomenon concerns a great diversity of molecules, which originate in human uses or activities releasing semi volatile organic compounds such as tire wear additives or perfluorinated compounds (PFAS). On one side, tire wear particles are a new focus as a form of environmental microplastics, with their role in air pollution expected to grow as tailpipe emissions decrease. On the other side, significant research currently focuses on PFAS due to the growing awareness of their toxicity and emerging regulations that often set their allowable concentrations at extremely low levels (a few parts per trillion, ppt). Despite this, many knowledge gaps remain regarding the environmental fate and transport of these compounds, often referred to as "forever chemicals" because of their environmental persistence. Given the limited reactivity of PFAS, a critical consideration is their partitioning behavior within environmental media. One particularly important aspect is the interaction between PFAS and microplastics, as these compounds are frequently found together due to their anthropogenic origins.
Goal of the project
The main goal of this PhD thesis is to investigate using computational kinetics the atmospheric degradation processes of emerging contaminants at the molecular level unraveling their most favorable pathways, their atmospheric fate and impact to the environment as well as their ecotoxicity towards aquatic species. The goal is to inform the experiments on recommended products to look for, and vice-versa, to support the kinetics and products already identified.
This project will also perform within the framework of a larger research program (CPER Ecrin; Labex CaPPA, and CDP AREA). This work will be conducted in close collaboration with the experimental works performed in the group led by Pierre Herckes at Arizona State University (USA).
Expected date of recruitment : 26/04/2025
Contact (e-mail address) : florent.louis()univ-lille.fr pierre.herckes()asu.edu sonia.taamalli()univ-lille.fr
Additional remarks/comments:
Candidate profile: Master’s degree or engineering degree in environmental chemistry of physical chemistry. Experience in the field of atmospheric chemistry, molecular simulations (quantum chemistry, molecular dynamics) and chemical kinetics will be appreciated. A good level of English (written/spoken) will be essential (at least B2). A mobility between the University of Lille and Arizona State University is mandatory. The work will take place at PC2A laboratory of the University of Lille.
PhD student: Avila ORTA
Supervisors: PETITPREZ Denis, HERBIN Hervé,
Financial support : CDP AREA / ED SMRE
Starting: Nov. 2025
Description of the subject: The aim of the thesis is to measure the optical properties of aerosols from biomass
burning aerosols (BBA) in the laboratory. These BBA aerosols are known to be one of the largest sources of
absorbing aerosols in the Earth's atmosphere, making them a key parameter in atmospheric chemistry and the
radiation budget. However, their great chemical and microphysical diversity makes them one of the most poorly
understood types of aerosol, and very difficult to observe and quantify using remote sensing. Moreover, BBA
aerosols are a major source of uncertainty for chemistry-transport and climate modelling.
The ambition of this thesis is therefore based primarily on the laboratory determination of the optical properties,
in particular the complex refractive indices (CRI) over a wide spectral range (from the far infrared to the
ultraviolet) of BBA aerosols for fuels representative of different types of vegetation. Measuring the CRI over such
a wide spectral range represents a real challenge and would be a major step forward in determining the optical
properties (lidar ratio, optical thickness, simple scattering albedo, spectral extinction, Ångström coefficient, etc.)
needed for remote sensing observations and climate modelling.
Work plan: As part of a previous thesis, experimental systems were set up in the PC2A laboratory to generate
particles from the 3 phases of biomass combustion: pyrolysis, open combustion and residual ash. The candidate
recruited will continue this experimental work, which involves recording the extinction spectra (FTIR and UV-
visible spectrometers) of BBA aerosols and characterising the physical and chemical properties of these particles
(particle counters, particle sizers, filter sampling for chemical analysis). In a second phase of the thesis project,
the recorded extinction spectra will be inverted in order to restore the ICRs using a numerical method developed
at the LOA. Any correlations between the optical properties of these aerosols and their chemical composition
will then be studied.
Scientific benefits : The experimental determination of the optical parameters that characterize the particles
emitted by biomass fires represents an expectation of the atmospheric science community because these data
are scarce but crucial for interpreting observations by remote sensing of these fires, the frequency and duration
of which intensity is likely to increase with climate change. They can also be injected into the inversion models
used by the teams working on the global scale modeling of carbonaceous aerosols.
Key words : Combustion aerosol metrology - Optical properties - Light scattering and absorption -
Fourier transform infrared and UV-visible spectroscopy - Atmospheric remote sensing
Scientific prrogram : Cross Disciplinary AREA (Aerosol at the Heart of the Earth-Atmosphere system.
PhD student: Muhammad BUTT
Supervisors: Laurent Gasnot, Luc-Sy Tran, Abderrahman El Bakali
Financial support: EGSMRE
Starting: Oct 2025
Increasing the share of biogas, a renewable and controllable energy source, in the energy mix is an important global objective. Although it is primarily composed of methane and CO2, it also contains traces of contaminants/impurities (sulfur, siloxanes, halocarbons, ammonia, and aromatics) that harm conversion systems and induce the formation of toxic pollutants. Biogas can also be used in neat form or in blends with other biofuels for greater efficiency. Understanding and controlling the combustion mechanisms and pollutant formation are essential for using biogas as a clean and safe alternative energy source, and thus promoting its use. In this context, this thesis project aims, for the first time, to contribute to a better understanding of the physicochemical phenomena involved in biogas combustion (alone and in a mixture with other biofuels) and in the formation of pollutants, considering the impact of contaminants.
This research project will draw on the internationally recognized expertise of the host laboratory. The highly sensitive experimental facilities of the laboratory will be used to analyze the key chemical species involved in biogas combustion. The PC2A laboratory (https://pc2a.univ-lille.fr/) has advanced premixed flame experimental benches that allow the work envisaged in this project to be carried out. The laboratory has made numerous analytical developments, including the implementation of gas chromatography methods to analyze chemical compounds present in very low concentrations (at the ppb level). Obtaining this detailed database, combined with the development of a representative kinetic model, will enable a detailed analysis of the reaction pathways managing biogas combustion (alone and in a mixture with other biofuels), taking into account the impact of contaminants, thus improving the design of reactors using this fuel, making them cleaner and more efficient.
Keywords: biogas, contaminants, pollutant emissions, flame, combustion, chemical kinetics, gas chromatography
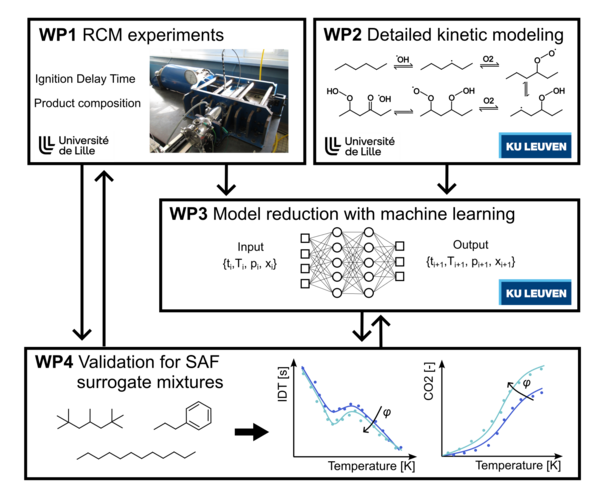
PhD Student: Valentina Yap-Celedon
Supervisors: Guillaume VANHOVE (PC2A, ULille), Florence VERMEIRE (CREaS, KULeuven)
Funding: Global PhD Fund (KULeuven/ULille)
Beginning: March 2025 (4 years)
Combustion-driven processes are still responsible for a large proportion of energy production and conversion worldwide. Aeronautic transport, especially, is one of the fastest-growing emission sources of Greenhouse Gases, causing sustainability concerns. Because aircraft electrification is not realistic in the coming decades, Sustainable Aviation Fuels have emerged as an immediate improvement in terms of climate change impact. The introduction of such fuels relies heavily on the possibility to model their combustion chemistry within the Computational Fluid Dynamics codes used in the industry. The chemical structure of such fuels, along with the wide range of temperatures, compositions, and pressures relevant to combustion in gas turbines, imposes the use of detailed kinetic models that contain tens of thousands of elementary reactions involving thousands of species. This results in unrealistically computationally expensive simulations, and has motivated the development of kinetic model reduction strategies for conventional (fossil-based) Jet-A fuels.
These strategies, however, frequently result in a loss of the predictive ability of the models. This project aims at developing, for the first time, robust and accurate kinetic models for Sustainable Aviation Fuels by means of a novel approach that integrates experiments, kinetic modeling, and machine learning. This approach benefits from the unique combined expertise of the ULille group in experimental work and kinetic modeling, and of the KULeuven group on kinetic modeling and machine learning. Experiments will be performed in Lille using a Rapid Compression Machine in the Low Temperature Combustion regime to validate detailed kinetic models for pure components as well as surrogate mixtures. These detailed kinetic models will then be used to train a ML model with a transfer learning methodology.
This project benefits from a 4-year PhD grant in co-direction between ULille and KULeuven, with stays in both labs over the grant’s duration, for an approximate duration of 3 years in Lille and 1 year in Leuven.
Keywords: Sustainable Aviation Fuels; Combustion chemistry; Kinetic modeling; Machine&Transfer Learning; Ignition delay.
Academic Requirements: A Master’s degree or an Engineering Degree in the fields of Chemistry, Chemical Engineering, or Mechanical Engineering and a taste for experimental work and computation are required. Additional knowledge in the fields of combustion chemistry will be beneficial.
Doctoral School: ED Sciences de la Matière, du Rayonnement et de l’Environnement (https://edsmre.univ-lille.fr)
About PC2A
PC2A (Physico-Chimie des Processus de Combustion et de l’Atmosphère) is a joint laboratory of the CNRS and the University of Lille, in which transdisciplinary research has been performed for more than 60 years in the fields of combustion and atmospheric chemistry. Based on a strong interaction between experimental and modeling work, the researchers in PC2A crave at building better understanding of the science behind the challenges of the current society, such as clean and safe energy, and the mitigation of, and adaptation to climate change.
About CREaS
Our core activities are related to (bio)Chemical Reactor Engineering and Safety. We aim to innovate (bio)chemical reactor technologies for a few carefully selected and industrially relevant target areas. Our goal is to push the boundaries of our discipline and increase the performance and safety of (bio)chemical processes.
With the Department of Chemical Engineering, we aim to provide a balance between creative fundamental and applied research by integrating expertise, methodologies, and techniques from different domains in chemical engineering. These range from analyzing micro-to macroscopic process through modeling and design, optimization and control, and advanced experimentation. Given the prevalence and economical importance of the chemical process industry in Belgium, the societal role of our department in the education and training of the next generation of chemical engineers is very important.
At KU Leuven, we offer a competitive and international working environment with access to the latest technologies and expertise. At our university, we commit to create an inclusive, respectful, and safe environment. KU Leuven ranks among the top 10 universities in Europe (top 50 worldwide) in the major university rankings.
PhD student: Madhumita Chakraborty
Supervisors: F. Louis, S. Taamalli
Financial support: CaPPA / ED SMRE
starting : Oct. 2024
The presence of atmospheric contaminants results either from direct emission from point sources: incineration, use of fossil fuels, industrial activities, etc., or from diffuse emissions or re-emissions from contaminated soils or waterbodies. This phenomenon concerns a great diversity of molecules, which originate in human uses or activities releasing volatile organic compound, containing a variety of different heteroatoms constituting a large category of emerging contaminants (pesticides, plastics, tire wear additives, PFAS, etc.). For many of them, the atmospheric pathway is their main mode of dispersion.
The primary route for the removal of contaminants from the atmosphere is by dry or wet deposition techniques. The chemical reaction initiated by atmospheric oxidants (OH, O3) are responsible for their transformation in the atmosphere. The products formed from these reactions may be hazardous and may lead to several negative implications. Deposited atmospheric fluxes of contaminants and corresponding degradation products also constitute an ecological risk for marine or terrestrial hydrosystems, apart from the potential human health risk induced by their presence in ambient air.
The main goal of this thesis is to investigate their atmospheric degradation processes using different theoretical approaches unravelling their most favourable pathways and their atmospheric fate and impact to the environment. As an add-on, the evaluation of the ecotoxicity for organic contaminants will be carried out in the aqueous environment.
This project will also perform within the framework of a larger research program (CPER Ecrin; Labex CaPPA, and CDP AREA). This work will be conducted in close collaboration with two experimental groups located in Canada (Toronto Metropolitan University and Concordia University in Montréal).
Applicants must have a master's degree in chemistry-physics or equivalent. Experience in the field of atmospheric chemistry, molecular simulations (quantum chemistry, molecular dynamics) and chemical kinetics will be appreciated. The work will take place at PC2A laboratory of the University of Lille.
Program connected to: CPER Ecrin, Labex CaPPA, CDP AREA
keywords: contaminants, atmosphere, reactivity, ecotoxicity, molecular simulations
PhLAM : Céline Toubin celine.toubin()univ-lille.fr
PhD student: Romie Massoud
Supervision: B. Hanoune / S. Crumeyrolle / L. Dauchet
Financial support: CaPPA / Région Hauts de France
Beginning: Oct. 2024
Air quality is a major environmental and health issue. Its health and economic impacts have been and continue to be the subject of numerous studies. On the public health front, since the early 2000s, work has been underway to gain a better understanding of air quality, both in ambient outdoor air and, more recently, in indoor air, and to identify effective solutions for reducing pollutants. For several years now, this subject has been a regular topic of public debate.
Epidemiological and public health studies show that exposure to air pollutants is associated with increased mortality and morbidity. But these studies use outdoor air measurements extrapolated to people's homes as a proxy for exposure, in the absence of actual measurements. This gives rise to major potential biases, due to local sources not taken into account by air quality monitoring networks, or more seriously, to exposure in confined environments, and to the rapid temporal variability of certain pollution phenomena. Miniature sensors, such as those developed as part of the University of Lille's APOLLINE project, provide access to this real-life exposure, and thus revisit the conclusions of epidemiological studies.
The objectives of this thesis project are to :
- Use portable miniature sensors to measure people's exposure to outdoor air in urban and peri-urban environments (MEL territory), in buildings (housing, work, leisure) and on public transport
- assess the determinants of indoor air pollution, a little-known area where we spend 90% of our time
- Propose recommendations for future large-scale epidemiological studies, combining ground-based data from sensors and reference stations.
Candidates should have a M2 degree in atmospheric chemistry, environmental sciences, analytical physicochemistry... A strong data analysis component is expected.
The thesis will be directed by B. Hanoune (PC2A) and co-supervised by S. Crumeyrolle (https://www-loa.univ-lille1.fr/). It will be carried out mainly at PC2A and LOA.
Keywords : air quality, indoor pollution, outdoor pollution, particulate matter, personal exposure.
Contacts :
benjamin.hanoune()univ-lille.fr
suzanne.crumeyrolle()univ-lille.fr
Financing envisaged : Région Hauts-de-France, ADEME, Université de Lille
PhD Student: Rana Shebly
Supervisors: Guillaume Vanhove, Yann Fenard
Financial support: ADEME / Region Hauts de France
starting: Oct. 2024
Background:
The reduction of greenhouse gas emissions - including CO2 and methane - is envisaged through the use of hydrogen instead of natural gas or even liquid fossil fuels. The production, storage and use of hydrogen from renewable energy sources is currently one of the challenges facing the mastery of green energies:
- During its production and storage phases, hydrogen, due to its very low collision cross-section, is quick to diffuse through materials, and so a rise in hydrogen levels in the atmosphere is expected if its use as a green energy carrier becomes widespread. This effect poses a problem for air quality, by modifying the reaction processes of atmospheric compounds. This may even run counter to the benefits of reducing greenhouse gases by replacing fossil fuels with hydrogen, by modifying the formation/consumption pathways of these greenhouse gases.
- In addition, hydrogen is known for its low-energy ignition hazard and its potential to transition from a deflagration to a detonation highly damaging to both infrastructure and people. Hydrogen's flammability limits are difficult to measure in any location or situation.
- Hydrogen combustion is well known for high temperatures, in excess of 600°C. However, for very low intermediate combustion temperatures, between 150 and 600°C, experimental data are almost non-existent. An excellent understanding of combustion phenomena in this temperature range is essential for accurate risk assessment, as well as for the efficient design of combustion systems.
These issues overlap: atmospheric chemistry and combustion are based on the same scientific foundations, with shared chemical reactions, thermodynamic equilibria and transport properties. However, temperature and pressure conditions differ. The challenge is to know precisely the rate constants, thermodynamic data and transport data of the chemical species involved over very wide temperature and pressure ranges, so as to be able to predict the reactivity and influence of hydrogen through detailed kinetic models.
Proposed study:
The Physical Chemistry of Combustion Processes and the Atmosphere Laboratory has been a center of expertise for 40 years on atmospheric reactions and combustion conditions. In 2023, the laboratory's combustion teams are involved in the PEPR hydrogène "Understanding and MOdeling NOx formation in Turbulent HYdrogen flames" (MONTHY) and are collaborating with members of the PEPR hydrogène "Améliorer les connaissances en matière de sécurité pour les mesures/modélisations de l'hydrogène en phase cryogénique" (ESKYMO) through a detailed kinetic model transfer. In addition, a partnership with General Electric has been set up to measure auto-ignition times of pure hydrogen or hydrogen mixed with natural gas over intermediate combustion temperature ranges. Finally, a thesis is in progress, with a defense scheduled for 2024, on the influence of hydrogen on the reactivity of molecules representative of the chemical natures present in fuels/biofuels.
On the basis of these experiments, the laboratory would like to initiate a thesis on the understanding of hydrogen reactivity under combustion conditions and atmospheric chemistry, to encompass the conditions of use of green hydrogen as an energy carrier. This approach is unprecedented in terms of the temperature range studied.
During this thesis, measurements of auto-ignition times will be carried out in a fast-compression machine. Hydrogen will be mixed with dimethyl ether, a highly reactive species, in order to achieve temperature conditions below 300°C. In addition, the laboratory is equipped with a cold-flame burner capable of operating between 30 and 600°C, thanks to enrichment of the reactive mixture with ozone (O3) and dimethyl ether (DME), which will enable hydrogen reactivity to be studied at temperatures as low as 150°C.
A detailed kinetic model validated under the conditions of this burner for a DME/O2/O3 mixture has also been developed as part of a thesis at the laboratory, and will enable an in-depth examination of the reactions specific to hydrogen under these conditions, free from the reactions specific to ozone and DME.
Results:
The experimental data collected will be used to refine our knowledge of hydrogen reactions, and to validate and advance the performance of a detailed kinetic model over a novel temperature range from ambient to adiabatic hydrogen flame temperature (approx. 2100°C). The modelling results obtained with a robust model will be used to estimate the hydrogen risk in its storage and use, the impact of hydrogen on air quality and, of course, the design of equipment for converting the chemical energy of hydrogen into useful energy.
The thesis will be directed by G. Vanhove and co-supervised by Y. Fenard.
Keywords : hydrogène, oxidation, réactivité homogène, combustion et atmosphère, modélisation cinétique
Contacts : yann.fenard()univ-lille.fr / guillaume.vanhove()univ-lille.fr
Financement envisagé : Région Hauts-de-France, ADEME, Université de Lille
PhD Student : Emilie Chantraine
Supervisor : Coralie Schoemaecker
Financial support : Région Hauts de France / ED SMRE*
Starting : Oct. 2023
The transformations of atmospheric pollutants emitted by human activities and present in the atmosphere and in indoor environments is a key question because of their impact on health, environment and climate change.
Indeed, numerous gaseous pollutants, mainly volatil organic compounds (VOC) are oxides by radiclas (highly reactive chemical species) such as OH (hydroxyl radical). This process generates more oxidised compounds, potentially more hazardous that the primary emitted species and heavier compounds leading to secondary organic aerosols (SOA).
This cycle is also responsible, in presence of nitric oxide (NO, from combustion processes), of the tropospheric ozone formation having a negative impact on health, plants and which is a Green House Gas (GHG).
In low NO environments, the oxidation processes are less known because radical-radical reactions are involved and are complex to study.
The work proposed in this thesis is based on an experimental approach of the oxidation processes using a characterisation technique of atmospheric radicals OHand HO2, both involved on these processes. The chemical reactivity of target species identified thanks to the European COST action INDAIRPOLLNET will be studied by the FAGE technique (Fluorescence Assay by Gas Expansion). This technique based on laser diagnostic is selective and sensitive and only used by about 10 laboratories in the World.
Expected date of recruitment : 01/10/2023
Contact (e-mail address) : coralie.schoemaecker@univ-lille.fr
Additional remarks/comments: application ADEME to submit, candidat for the PhD should be identify for the application, deadline end of March. If interested please contact us ASAP
PhD student: Mohammad ISSA
Supervisors: Pascale Desgroux, Luc-Sy Tran
Financial support: PEPR OXY3C
Context:
This PhD thesis is part of the ambitious program “Support innovation to develop new largely carbon-free industrial processes” supported by the French Government in the framework of the decarbonization of the industry to achieve carbon neutrality by 2050.
The decarbonation of the industry partly relies on the development and intensification of processes for CO2 capture. This PhD thesis is offered as part of the OXY3C project aiming at improving knowledge and skills in oxycombustion for the optimization of eco-efficient processes. The consortium working on this project gathers seven French academic laboratories and IFPEN.
Objectives of the thesis:
This study aims at investigating chemical kinetics of soot precursors and measuring soot particles from biomass’s tar surrogates in the framework of a close collaboration between PC2A, LRGP, and IFPEN. Experiments and simulations will be performed under CO2 and H2O vapor atmosphere at a wide range of temperatures covering the temperature range in the Chemical Looping Combustion application. This work comprises two main parts.
The first part is establishing a reliable experimental database that includes mole/volume fraction profiles of reactants, products, intermediates and soot particles in the pyrolysis and combustion of a biomass’s tar surrogate. These data will be measured in a burner system at PC2A and in a jet-stirred reactor or a tubular reactor at LRGP. Chemical species will be measured using gas chromatography and mass spectrometry. Soot volume fraction profiles in flames will be measured in situ by extinction or cavity ring-down extinction. Selected soot samples thermophoretically collected in the above flames will be analyzed at IFPEN for characterizing soot morphology using microscopy (STEM + possibly TEM or HRTEM).
The second part is developing a detailed kinetic model for soot precursor formation from the pyrolysis and combustion of this biomass’s tar surrogate. The model will include a base model containing common species, a sub-model for the pyrolysis and combustion of a biomass’s tar surrogate, a sub-model of aromatics. The model will be tested against the experimental database under CO2 and H2O atmosphere that will be obtained in the above experimental part. The model will be transferred to IFPEN for soot modeling.
PhD student : Luna Cartayrade
Supervisor: Florent Louis and Nadine Borduas-Dedekind (Université de Colombie Britannique, Vancouver, Canada)
Financial support : Université de Lille - thèse labellisée Graduate Program / Labex CaPPA
Starting : Oct. 2023
Selenium (Se) is an essential dietary nutrient. Its biogeochemical cycle, including its atmospheric component, influences animal and human health through biological processes that regulate our daily lives, like the immune system and thyroid function. The recommended dietary intake range of Se is narrow, with safe daily intake levels between 20 to 450 μg for adults. Humans and animals obtain Se from their diet, wherein the Se content of plant-based foods depends on the amount of bioavailable Se in agricultural soils. Atmospheric deposition is a major source of Se to
agricultural soils, and atmospheric cycling of Se is a potential driver for soil Se distribution.
Atmospheric Se is thought to have four main sources: anthropogenic, marine biosphere, terrestrial biosphere, and volcanic emissions. Reduction with subsequent methylation by microorganisms of inorganic Se play an important role in the formation of volatile Se species, such as methane selenol, dimethyl selenide or dimethyl diselenide found in marine systems. Volatilization can lead to gas-phase emission of such organo-selenides from the aquatic phase. It is generally postulated that organic Se compounds have a rather short lifetime in the atmosphere, where they are being rapidly oxidized and consequently partition to the particle phase. However, the available information is preventing us from predicting the chemical speciation and the fate of volatile organic Se compounds in the atmosphere. With changing climates, there is an urgent need to model Se’s biogeochemical cycle in order to mitigate future Se deficiencies.
In addition to the experiments that will be performed at the University of British Columbia, this thesis project will evaluate the fate of Se-containing species by using computational chemistry tools to
elucidate the stepwise chemical oxidation mechanism of atmospheric Se to enable the predictive capabilities of the fate of these compounds for future Se-soil distribution maps. Ultimately, this project will provide insight into currently unknown aspects of gas phase oxidation of selenium leading to an improved modeling of the fate of Se and its impact on soil distribution in a changing climate.
In recent decades, theoretical kinetics has contributed significantly to a better understanding of a significant number of atmospheric reactions and has made it possible to estimate the associated rate constants with chemical accuracy. This improvement has been the result of important advances in computational tools and the precision of theoretical methods (i.e., theories of electronic structure and chemical kinetics). A systematic benchmark study of the suitable theoretical methods will be performed for assessing different properties such as for example geometrical parameters, vibrational frequencies, and bond strengths for selenium-containing species. In a second step, this project aims to shed some light in the understanding of the experimental facts and to determine both thermochemical properties and kinetics parameters for the commonly studied molecular systems. The goal is to inform the experiments on recommended products to look for, and vice-versa, to
support the kinetics and products already identified. The use of theoretical kinetics will also be helpful to determine all reaction channels whenever the products could not be identified in the
experiments. These results will provide new knowledge on the fate of Se compounds in the atmosphere in order to assess their atmospheric transport, their fate, and ultimately their spatial distribution to
assess future Se deficiencies.
Supervisors: Florent Louis and Nadine Borduas-Dedekind (Université de Colombie Britannique, Vancouver, Canada)

