Masters
Faire son stage de Master2 au laboratoire
propositions pour l'année universitaire 2023-2024
Les polluants atmosphériques ont des effets reconnus à court et long terme sur la santé humaine. A titre d’exemple, les particules fines (< 2.5 µm de diamètre) réduiraient en moyenne l’espérance de vie des Français de plus de 9 mois. Les niveaux de pollution mesurés dans les grandes villes dépassent régulièrement et largement les normes règlementaires, et les mesures réalisées par le réseau ATMO au niveau national ont montré que la métropole lilloise se classe 3ème ville la plus polluée de France. Cependant, ces mesures, réalisées par un nombre très réduits de stations fixes (2 seulement sur tout le territoire de la MEL, de 142 km2) ne reflètent pas la variabilité spatiale et temporelle des concentrations de polluants.
Pour prendre en compte cette variabilité, le groupe de travail multilaboratoires de l’Université de Lille APOLLINE (Air pollution and individual exposure – www.apolline.science), a mis en place un réseau de capteurs miniatures à bas coût. D’une part, des capteurs fixes peuvent être installés, en extérieur comme à l’intérieur de bâtiments, pour des mesures à long terme de polluants gazeux et particulaires. D’autre part, des capteurs mobiles de particules peuvent être distribués à des volontaires pour établir des cartes de pollution à haute résolution spatiotemporelle, et pour évaluer leur exposition.
Deux sujets différents peuvent être traités lors de ce stage :
- L’étude de l’exposition individuelle aux particules de volontaires sur le territoire de la MEL
- Une étude préliminaire de l’exposition des pompiers aux polluants gazeux et particulaires lors de leurs entraînements et pendant les interventions.
Les deux sujets impliquant de travailler avec du public, ce stage est réservé aux étudiants francophones.
Key words: pollution de l’air, capteurs, sciences participatives, mesures de terrain, analyse de données
Supervisor: CRUMEYROLLE Suzanne (suzanne.crumeyrolle()univ-lille.fr) laboratory LOA
Collaborator: HANOUNE Benjamin (PC2A)
Chemical emissions from tire wear become an emerging environmental concern because tires are made of a complex mixture of rubber and various chemical additives. As tires wear down, these compounds can be released into the environment. Some of these compounds can have toxic or harmful effects on organisms in aquatic ecosystems. While N-(1,3-Dimethylbutyl)-N'-phenyl-p-phenylenediamine (6PPD) used in tires has caused environmental concern, antioxidants used in tires keep tires strong, flexible, and long-lasting and are essential for the safety of vehicles. Antioxidants added in tires can prevent the rubber from degrading due to oxidation, which can cause the rubber to become brittle and weak, resulting in premature tire failure and road safety issues. The group of p-phenylene-diamine antioxidants (PPDAs) are less well studied in terms of their environmental fate processes (transport and transformation) and impact.
The overall objective of this internship is to explore the chemistry (reactivity, reaction pathways and kinetics) related to the effectiveness of the chemical function in PPDAs and environmental risks after being released to the environment.
This project will also aim to contribute to a larger research program devoted to the study of atmospheric processes (Labex CaPPA, CPER Ecrin). The work will take place at PC2A laboratory, Lille University.
Key words: molecular simulations, atmosphere, tire wear, reactivity
Supervision : Florent LOUIS (florent.louis()univ-lille.fr, tel : 0320336332), Sonia TAAMALLI
Research project S3*; The S3 project is mainly dedicated to bibliographical research, preparation of a future laboratory work, feasibility tests in agreement with the laboratory policies (No internship agreement will be signed for the S3 project). The payment of a bonus is not mandatory.
Research project S4*; The S4 project is a full time research training (5-6 months) and an internship agreement should be signed. Students must receive an internship bonus in accordance with university policies. Supervisors must provide the internship bonus.
* Preferably, both options will be selected. However, the student is free to choose another project in S4.
Melamine is an s-triazine with three amino groups and is mainly used to synthesize melamine-formaldehyde (MF) resins which are added to kitchenware, textiles, foamed plastics, electric appliances, and flooring to impart fire resistance. Aside from MF resins, melamine is also used in paints and coatings, flooring, machine wash liquids, leather treatment products and is commonly used as material for baby and children cutlery and plates. It is a high production-volume chemical with an import volume of up to 1000000 tons per year into the EU.
Melamine has been recently suggested as a persistent, mobile and toxic (PMT) substance and has gained increasing public attention worldwide. In order to protect public health and aquatic ecosystems and improve water quality, it is essential to understand the occurrence and fate of melamine and its related triazines (i.e., atrazine, cyromazine, ammeline, etc.) in waters.
The overall objective of this internship is to explore the chemistry (reactivity, reaction pathways and kinetics) related to the atmospheric degradation of melamine in the aqueous phase.
This project will also aim to contribute to a larger research program devoted to the study of atmospheric processes (Labex CaPPA, CPER Ecrin). The work will take place at PC2A laboratory, Lille University and will be performed in collaboration with Prof. Roxana Suehring from Toronto Metropolitan University in Canada.
Key words: molecular simulations, atmosphere, melamine, reactivity, water
Supervision: Florent LOUIS (florent.louis()univ-lille.fr, tel : 0320336332), Collaborator: Roxana SUEHRING / Toronto Metropolitan University (Canada)
Research project S3*; The S3 project is mainly dedicated to bibliographical research, preparation of a future laboratory work, feasibility tests in agreement with the laboratory policies (No internship agreement will be signed for the S3 project). The payment of a bonus is not mandatory.
Research project S4*; The S4 project is a full time research training (5-6 months) and an internship agreement should be signed. Students must receive an internship bonus in accordance with university policies. Supervisors must provide the internship bonus.
* Preferably, both options will be selected. However, the student is free to choose another project in S4.
Radicals play an important role in the atmosphere, controlling its oxidative capacity. Characterizing the reactions of radicals in the atmosphere is an ever present challenge. Spectroscopic methods have proven successful in measuring radicals in different reactors through their UV-visible or infrared spectra. However, rotational spectroscopy would allow absolute specificity in determining the identity of radicals as well as molecules from experiment. The distinguishing factor of rotational spectroscopy over other techniques is in its absolute determination of structural isomers, such as CH3O and CH2OH, which have the same mass but entirely different rotational spectra.
The Fast Flow Tube apparatus in PC2A/CERLA will be combined with the Chirped Pulse Fourier Transform Millimeter-wave Spectrometer in PhLAM (Figure 1) to address two scientific goals: 1) Measure the spectra of Radicals with Large Amplitude Motion (RLAMs) and 2) Measure product branching ratios of chemical reactions. RLAMs are represented in most organic radicals, as many contain a -CH3 moiety that can rotate, causing tunneling splitting from the large amplitude motion. This large amplitude motion challenges theoretical descriptions, including the prediction of chemical reactions rates from ab initio calculations. RLAMs are prominent as reactants and products in the atmosphere, with the methyl peroxy (CH3O2) serving as a prime example. Most RLAMs rotational spectra are unknown though, so the collaboration between PC2A and the SPECTRO team in PhLAM will search for these spectra relating it to their structure and then search for them as products of reactions of atmospheric relevance.
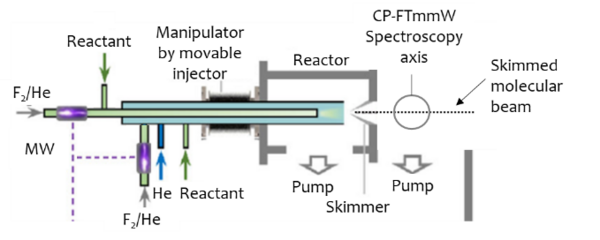
Figure 1: Schematic of the combination of the Fast Flow Tube and Chirped pulse Fourier transform Millimeter-wave spectrometer
The radicals will be generated in the flow tube reactor using microwave discharges of fluorine (or chlorine) and reactions with precursors, they will then be reacted with oxygen to make peroxy radicals or new reaction products. The laminar flow of this reactor and radicals generation possibilities give ideal conditions to control reactions before expansion into a differentially pumped chamber, where the radicals would be probed at lower pressures more conducive to rotational spectroscopy experiments. The chirped pulse Fourier transform Millimeter-wave spectrometer from PhLAM ranging from 50-500 GHz will be used to probe a variety of molecules and radicals, and determine relative branching ratios. Spectroscopy will first be proven on ideal systems and then applied to reactions of atmospheric interest.
This project is addressed to candidates interested in the experimental aspects of the research and having knowledge in atmospheric chemistry and/or spectroscopy.
Keywords: Atmospheric Chemistry, Spectroscopy, Oxidation, Radicals
Supervisors: PILLIER Laure (laure.pillier()univ-lille.fr, HdR, PC2A) , HAYS Brian (brian.hays()univ-lille.fr, PhLAM)
Eventually CaPPA Work Package: WP-1 From gas phase to aerosols
Les Masters en cours
Student : Rana Shebly
Because of the share of thermal power plants in the worldwide energy mix, the mitigation of the effect of power generation on the emission of greenhouse gases is of timely importance. The use of “green” hydrogen as a fuel is widely regarded as one of the impactful existing solutions to preserve the production of electricity in the short-term. It is therefore the subject of combustion studies that evaluate the impact of hydrogen addition on the fundamental combustion properties, such as burning velocity, pollutant formation or ignition delay.
In the high pressures and moderate temperatures representative of the auxiliaries of gas turbines, one must ensure that adding hydrogen does not result in increased safety risks. The wide flammability limits, high laminar burning velocity can indeed be problematic when flame conditions are created. To evaluate this possibility, a collaborative study has been initiated between the PC2A laboratory and General Electric.
During this internship, the selected candidate will perform experiments using the ULille Rapid Compression Machine to measure the ignition delays of blends of natural gas and hydrogen in well-controlled conditions representative of the auxiliaries of gas turbines: high pressures, temperatures below 1000 K, and wide mixture compositions ranging from very fuel-lean to highly fuel-rich. The obtained experimental results will be compared to simulation results obtained using the state-of-the-art kinetic models from the literature, to demonstrate which model is the most valid in such conditions, and propose improvements to such models.
Keywords: Hydrogen, natural gas, ignition delay times, combustion, gas turbines
Contact e-mail: guillaume.vanhove@univ-lille.fr, yann.fenard@univ-lille.fr

